Varoglutamstat Inhibits the Dimerization of the Aβ25–35 Fragment in Aqueous Solution
Chúng tôi vui mừng thông báo rằng TS. Ngô Sơn Tùng và các đồng nghiệp gần đây đã xuất bản công trình của họ có tựa đề "Varoglutamstat Inhibits the Dimerization of the Aβ25–35 Fragment in Aqueous Solution" trên tạp chí The Journal of Physical Chemistry B
Tóm tắt:
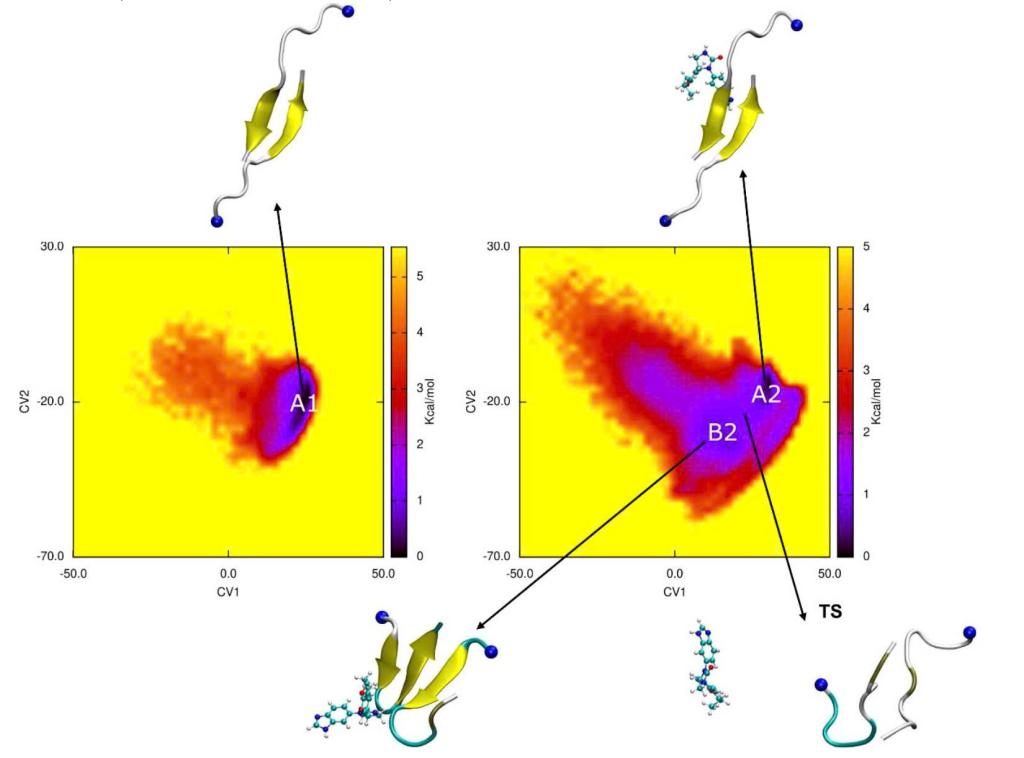
The self-aggregation of amyloid-beta (Aβ) peptides is strongly associated with Alzheimer’s disease. In this study, the influence of the small varoglutamstat compound on the conformations of the FAβ25–35 dimer was extensively characterized by using MD simulations. The influence of the ligand on the conformation of the FAβ25–35 dimer was studied during the first 10.0 μs. However, its real influence was clarified when the trajectory was extended to 20.0 μs. This indicates that the investigation of a ligand inhibiting Aβ requires a longer MD simulation than previously thought. The ligand changes ensemble properties by reducing the formation of nonbonded intermolecular contacts and β-content. Although varoglutamstat has weak binding to the FAβ25–35 dimer, the free energy landscape is impacted in shape, free energy barrier, and number of minima. Notably, it is found that the population of the amyloid-competent dimer structure is substantially reduced upon ligand addition. Furthermore, the change from β-hairpin conformation to antiparallel structure may occur through a transition state forming a random coil conformation.
- Log in to post comments
