Aβ42 Adopts a Stronger Binding Affinity to the Gold Surface than Aβ40
We are pleased to announce that Dr. Ngo Son Tung and MSc. Thai Quynh Mai recently published their work titled "Aβ42 Adopts a Stronger Binding Affinity to the Gold Surface than Aβ40" in the journal The Journal of Physical Chemistry B.
Abstract:
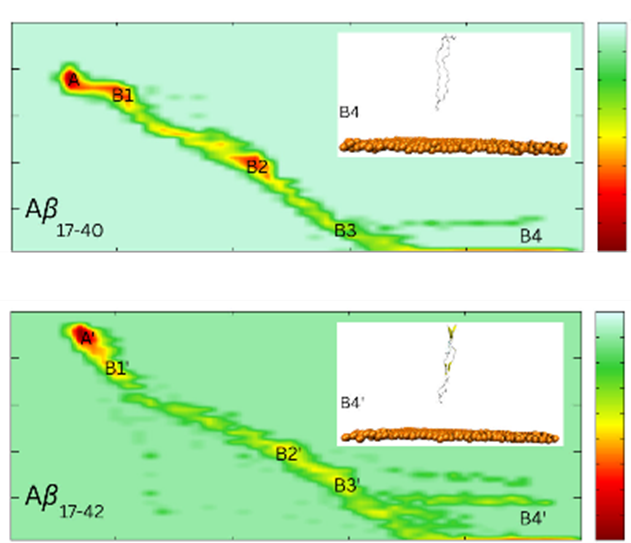
Soluble Aβ oligomers are categorized as major agents of Alzheimer’s disease progression, instead of insoluble fibrils. The binding affinity of Aβ peptides on the gold surface is associated with the biocorona due to the Vroman effect. This phenomenon can be used to screen and/or remove highly toxic amyloid pieces using gold nanoparticles. In this context, the binding process of Aβ40 and Aβ42 dimers to the gold nanosurface was investigated via atomistic simulations, including molecular dynamics (MD) and steered-MD (SMD) simulations. In particular, the obtained results indicate that the Aβ17–42 dimer exhibits a stronger binding affinity to the gold surface than the Aβ17–40 dimer in terms of the rupture force, pulling work, and Jarzynski’s free energy analyses. During this process, the van der Waals (vdW) interaction energy plays an important role, and Aβ17–42 adopts a significantly larger value compared with Aβ17–40. The enlarged interaction is caused by the additional hydrophobic residues at the C-terminus, including Ile41 and Ala42. Furthermore, free energy landscape outcomes demonstrate that Aβ17–42 maintains a rigid β-hairpin during the dissociation process, while Aβ17–40 becomes a random coil.
- Log in to post comments
